Details of the Drug
General Information of Drug (ID: DMKIML1)
| Drug Name |
D-tryptophan
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
D-Tryptophan; 153-94-6; H-D-Trp-OH; D(+)-Tryptophan; (R)-Tryptophan; D-TRYPTOPHANE; (2R)-2-amino-3-(1H-indol-3-yl)propanoic acid; D-Trytophane; H-D-Typ-OH; Tryptophan, D-; D-Trp; UNII-7NS97N9H1G; D-(+)-Tryptophan; (R)-2-Amino-3-(3-indolyl)propionic acid; EINECS 205-819-9; NSC 97942; (R)-(+)-2-Amino-3-(3-indolyl)propionic Acid; 7NS97N9H1G; CHEMBL292303; DTR; CHEBI:16296; QIVBCDIJIAJPQS-SECBINFHSA-N; MFCD00005647; D-alpha-Amino-3-indolepropionic acid; NCGC00093372-02; DSSTox_RID_82038; DSSTox_CID_26989; D(+)-Tryptophan,
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
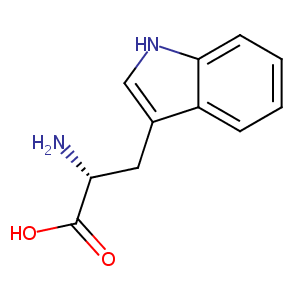 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 204.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
